Abstract
Introduction:
FIX(a) binds rapidly and reversibly to vascular endothelium and extravascular matrix, in part mediated by the interaction of specific Gla domain residues with collagen IV. Both anticoagulant heparan sulphate and collagen IV localize predominantly to the basement membrane and supramolecular structure analysis suggests that heparan sulphate chains are integrated into collagen IV-containing networks. Previous work has demonstrated the contribution of the heparin and antithrombin (AT)-binding exosites in the protease domain to the regulation of human FIXa. Here we evaluate the contribution of these protease exosites to FIX hemostatic function and clearance in the hemophilia B (HB) mouse.
Methods:
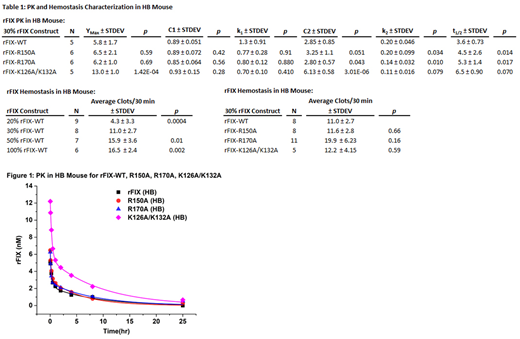
Recombinant human FIX variants expressed in HEK293 cells with substitutions in the heparin- (K126A/K132A or R170A) or AT-binding (R150A) exosites (chymotrypsinogen #) were purified to homogeneity. In vivohemostasis was assessed in a saphenous vein bleeding model in HB mice. Anesthetized adult mice were injected with a weight-based dose predicted to achieve 30% FIX plasma concentrations and the exposed left saphenous vein was punctured with a 29 gauge needle. Clot formation at the injury site was repeatedly disrupted over 30 min to determine the average time to clot formation and total #clots/30 min. FIX pharmacokinetics (PK) in HB mice were determined with a weight-based dose injected into the tail vein. Serial retro-orbital blood samples were collected, plasma isolated and FIX content determined with a human FIX specific ELISA. FIX concentrations were plotted versus time and initially fit to the equation: [IX]t = [IX]max*(C1*e(-k1*t)+ C2*e(-k2*t)), where k1 and k2 are rate constants for the initial and terminal elimination phases, respectively. As the 4-parameter fit resulted in some large error estimates, the two phases were fit separately to estimate the respective parameters.
Results:
Previous characterization of APTT-based coagulant activities for these FIX variants (% WT ± SEM) were as follows: FIX WT 100 ± 1.4, R150A 73.6 ± 1.4, K126A/K132A 20.6 ± 9.2, and R170A 609.2 ± 37. In the saphenous vein bleeding assay, HB mice had no clot formation over 30 min, while wild type mice demonstrated 11 clots/30 min. When HB mice were injected with increasing concentrations of FIX WT (20%-100%), a dose dependent increase was observed from 4.3 to 16.5 clots/30 min (Table 1). When injected with sufficient FIX to achieve 30% plasma levels, the AT-binding exosite variant FIX R150A and heparin binding site variant K126A/K132A demonstrated similar hemostatic activity to FIX WT in the HB mouse. FIX R170A (30%) trended towards enhanced activity relative to WT, while 10% FIX R170A levels demonstrated similar activity to 30% FIX WT (not shown).
The PK (5 min to 25 hr) of FIX was examined in HB and wild type mice. FIX WT demonstrated approximately ~30% recovery (initial peak) of predicted levels. FIX K126A/K132A had markedly enhanced recovery (2.2-fold higher than FIX WT), while FIX R150A and R170A demonstrated similar recovery to FIX WT (Table 1, Figure 1). FIX K126A/K132A demonstrated ~70% of predicted recovery. A rapid decrease in FIX levels was observed over the ensuing 4-6 hours with similar k1 values for all proteins. In contrast, terminal phase k2 values trended lower and predicted half-lives longer for the heparin-binding exosite mutants (FIX K126A/K132A and R170A). Consistent with occupation of extravascular sites by endogenous FIX, PK studies in wild type mice demonstrated ~20% enhancement of FIX recovery relative to HB mice (not shown).
Conclusions:
In contrast to in vitrocoagulant and thrombin generation activity, FIX K126A/K132A demonstrated similar in vivohemostatic activity to FIX WT, markedly enhanced recovery and modestly slower terminal clearance. The enhanced in vivo hemostatic activity of this variant relative to coagulant activity likely results from the markedly enhanced plasma FIX recovery. In contrast, FIX R150 demonstrates similar in vivo hemostatic activity and zymogen clearance to FIX WT. FIX R170A demonstrated ~3-fold enhanced hemostatic potency and similar zymogen clearance compared to FIX WT. This hemostatic potency is consistent with the enhanced coagulant activity of this FIX Padua (R170L) related variant. In addition to the collagen IV binding site, the FIX(a) heparin binding exosite is likely to play a substantial role in FIX localization to the extravascular compartment.
Sheehan:Pfizer: Research Funding; Bayer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal